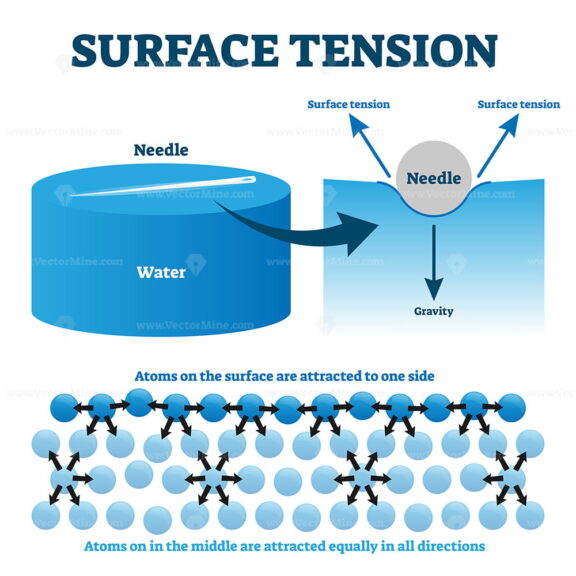
What Does High Surface Tension Mean . The cohesive forces between liquid molecules are responsible for the. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. This means that liquids with higher surface tension are less likely to mix with another. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. Water has a high surface tension because the water molecules on the surface are pulled together. This term is typically used only. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Generally, the higher the surface tension, the higher the interactions within the phase. Water consists of one oxygen atom flanked by two hydrogen atoms.
from vectormine.com
This term is typically used only. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Water consists of one oxygen atom flanked by two hydrogen atoms. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. The cohesive forces between liquid molecules are responsible for the. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. This means that liquids with higher surface tension are less likely to mix with another. Generally, the higher the surface tension, the higher the interactions within the phase. Water has a high surface tension because the water molecules on the surface are pulled together.
Surface tension explanation vector illustration diagram VectorMine
What Does High Surface Tension Mean Water has a high surface tension because the water molecules on the surface are pulled together. This term is typically used only. Water consists of one oxygen atom flanked by two hydrogen atoms. Water has a high surface tension because the water molecules on the surface are pulled together. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. The cohesive forces between liquid molecules are responsible for the. Generally, the higher the surface tension, the higher the interactions within the phase. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. This means that liquids with higher surface tension are less likely to mix with another.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments What Does High Surface Tension Mean Water has a high surface tension because the water molecules on the surface are pulled together. Water consists of one oxygen atom flanked by two hydrogen atoms. Generally, the higher the surface tension, the higher the interactions within the phase. This means that liquids with higher surface tension are less likely to mix with another. Surface tension, property of a. What Does High Surface Tension Mean.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts What Does High Surface Tension Mean This means that liquids with higher surface tension are less likely to mix with another. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. This term is typically used only. The cohesive forces between liquid molecules are. What Does High Surface Tension Mean.
From byjus.com
Explain the surface tension phenomenon with examples. What Does High Surface Tension Mean Water consists of one oxygen atom flanked by two hydrogen atoms. Generally, the higher the surface tension, the higher the interactions within the phase. The cohesive forces between liquid molecules are responsible for the. Water has a high surface tension because the water molecules on the surface are pulled together. This term is typically used only. Surface tension is a. What Does High Surface Tension Mean.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance What Does High Surface Tension Mean Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. This means that liquids with higher surface tension are less likely to mix with another. Water has a high surface tension because the water molecules on the surface are pulled together. The cohesive forces between liquid molecules are responsible for the.. What Does High Surface Tension Mean.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 What Does High Surface Tension Mean This means that liquids with higher surface tension are less likely to mix with another. The cohesive forces between liquid molecules are responsible for the. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water has a high surface tension because the water molecules on the surface are pulled together. Surface tension, property of. What Does High Surface Tension Mean.
From uk.onlinelabels.com
Surface Energy and Labels The Unscientific Guide What Does High Surface Tension Mean Water consists of one oxygen atom flanked by two hydrogen atoms. Water has a high surface tension because the water molecules on the surface are pulled together. Generally, the higher the surface tension, the higher the interactions within the phase. This means that liquids with higher surface tension are less likely to mix with another. The cohesive forces between liquid. What Does High Surface Tension Mean.
From study.com
Surface Tension Definition, Calculation & Examples Video & Lesson What Does High Surface Tension Mean This term is typically used only. The cohesive forces between liquid molecules are responsible for the. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water consists of one oxygen atom flanked by two hydrogen atoms. Generally,. What Does High Surface Tension Mean.
From www.biolinscientific.com
3 ways to measure surface tension What Does High Surface Tension Mean Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. This means that liquids with higher surface tension are less likely to mix with another. Water has a high surface tension because the water molecules on the surface are pulled together. Generally, the higher the surface tension, the higher the interactions within the. What Does High Surface Tension Mean.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint What Does High Surface Tension Mean Water consists of one oxygen atom flanked by two hydrogen atoms. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. This term is typically used only. This means that liquids with higher surface tension are. What Does High Surface Tension Mean.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID3227401 What Does High Surface Tension Mean Generally, the higher the surface tension, the higher the interactions within the phase. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. The cohesive forces between liquid molecules are responsible for the. This means that liquids with higher surface tension are less. What Does High Surface Tension Mean.
From www.thoughtco.com
Surface Tension Definition in Chemistry What Does High Surface Tension Mean This means that liquids with higher surface tension are less likely to mix with another. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Water consists of one oxygen atom flanked by two hydrogen atoms. The cohesive forces between liquid molecules are responsible for the. Liquids with high surface tension. What Does High Surface Tension Mean.
From www.slideserve.com
PPT Notes Water and its Special Properties PowerPoint Presentation What Does High Surface Tension Mean This term is typically used only. Water consists of one oxygen atom flanked by two hydrogen atoms. Generally, the higher the surface tension, the higher the interactions within the phase. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. This means that liquids with higher surface tension are less likely to mix. What Does High Surface Tension Mean.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint What Does High Surface Tension Mean Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Water has a high surface tension because the water molecules on the surface are pulled together. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Surface tension,. What Does High Surface Tension Mean.
From www.lceted.com
Tension Vs Compression Difference Between Tension & Compression What Does High Surface Tension Mean Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Water consists of one oxygen atom flanked by two hydrogen atoms. This term is typically used only. Water has a high surface tension because the water molecules on the surface are pulled together. Surface tension is a phenomenon in which the. What Does High Surface Tension Mean.
From slidetodoc.com
Lecture 14 Taken in part from Chapters 13 What Does High Surface Tension Mean Water consists of one oxygen atom flanked by two hydrogen atoms. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. The cohesive forces between liquid molecules are responsible for the. Surface tension, property of a liquid surface displayed by its acting as. What Does High Surface Tension Mean.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL What Does High Surface Tension Mean Water has high surface tension, which can be explained by its polarity and hydrogen bonding. This means that liquids with higher surface tension are less likely to mix with another. This term is typically used only. The cohesive forces between liquid molecules are responsible for the. Generally, the higher the surface tension, the higher the interactions within the phase. Water. What Does High Surface Tension Mean.
From www.researchgate.net
a wetting state of surface with low and highsurfacetension fluids b What Does High Surface Tension Mean Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. This means that liquids with higher surface tension are less likely to mix with another. This term is typically used only. Generally, the higher the surface tension, the higher the interactions within the. What Does High Surface Tension Mean.
From sciencing.com
What Is the Difference Between High & Low Surface Tension? Sciencing What Does High Surface Tension Mean Water has a high surface tension because the water molecules on the surface are pulled together. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. The cohesive forces between liquid molecules are responsible for the. Generally, the. What Does High Surface Tension Mean.